The Mac
@TheMac
26 September, 05:06
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
The Mac
@TheMac
26 September, 05:11
In response The Mac to his Publication
Father, forgive them; for they know not what they do.
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
The Mac
@TheMac
26 September, 05:14
In response The Mac to his Publication
COVID-19 and Brain Injury. COVID-19 may lead to changes in personality and behavior, which require treatment across the continuum of care. Traumatic brain injury, stroke, anoxic brain injury, encephalitis, and other neurological conditions leading to brain insult can be devastating to individuals and those close to them ( Table 1 ).
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
The Mac
@TheMac
26 September, 05:16
In response The Mac to his Publication
Brain injury due to oxygen deficiency Lightning or Electric shock
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
The Mac
@TheMac
26 September, 05:22
In response The Mac to his Publication
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
The Mac
@TheMac
26 September, 05:22
In response The Mac to his Publication
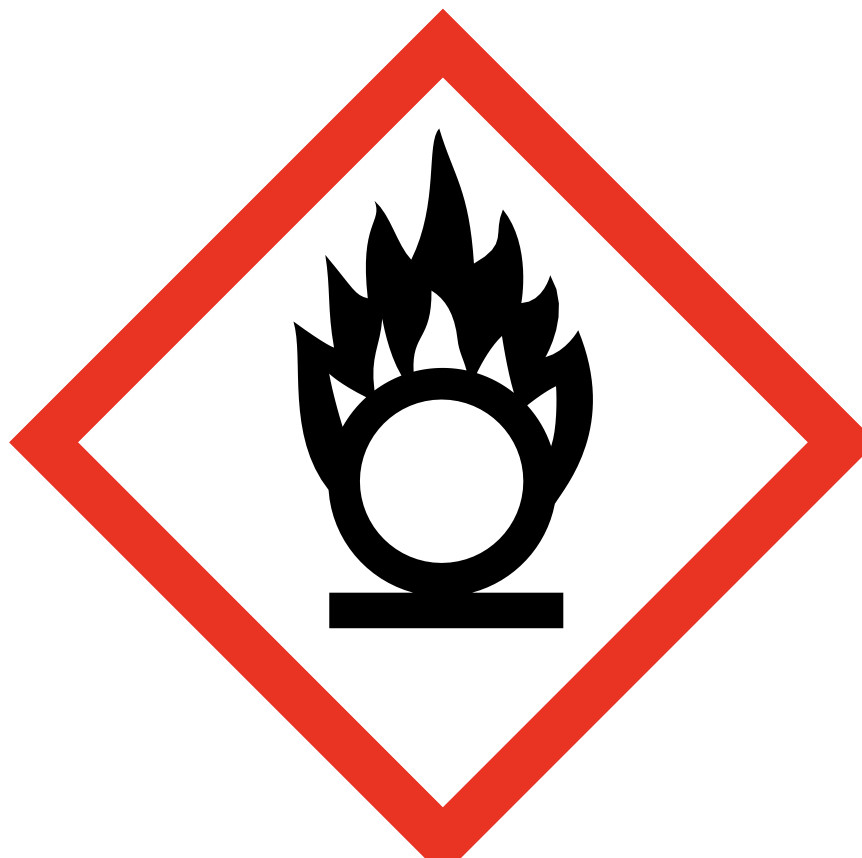
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent (called the reductant, reducer, or electron donor). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
The Mac
@TheMac
26 September, 05:23
In response The Mac to his Publication
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, to a substrate. Combustion, many explosives, and organic redox reactions involve atom-transfer reactions.
05:24 PM - Sep 26, 2022
In response The Mac to his Publication
Only people mentioned by TheMac in this post can reply
The Mac
@TheMac
26 September, 05:25
In response The Mac to his Publication
In a combustion reaction, a fuel is heated and it reacts with oxygen. The fire triangle summarises the three things needed for combustion - a fuel, heat and oxygen.
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
The Mac
@TheMac
26 September, 05:26
In response The Mac to his Publication
In physics, a photon gas is a gas-like collection of photons, which has many of the same properties of a conventional gas like hydrogen or neon – including pressure, temperature, and entropy. The most common example of a photon gas in equilibrium is the black-body radiation.
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396