Dennis 369
@Dennis369
23 August, 10:05
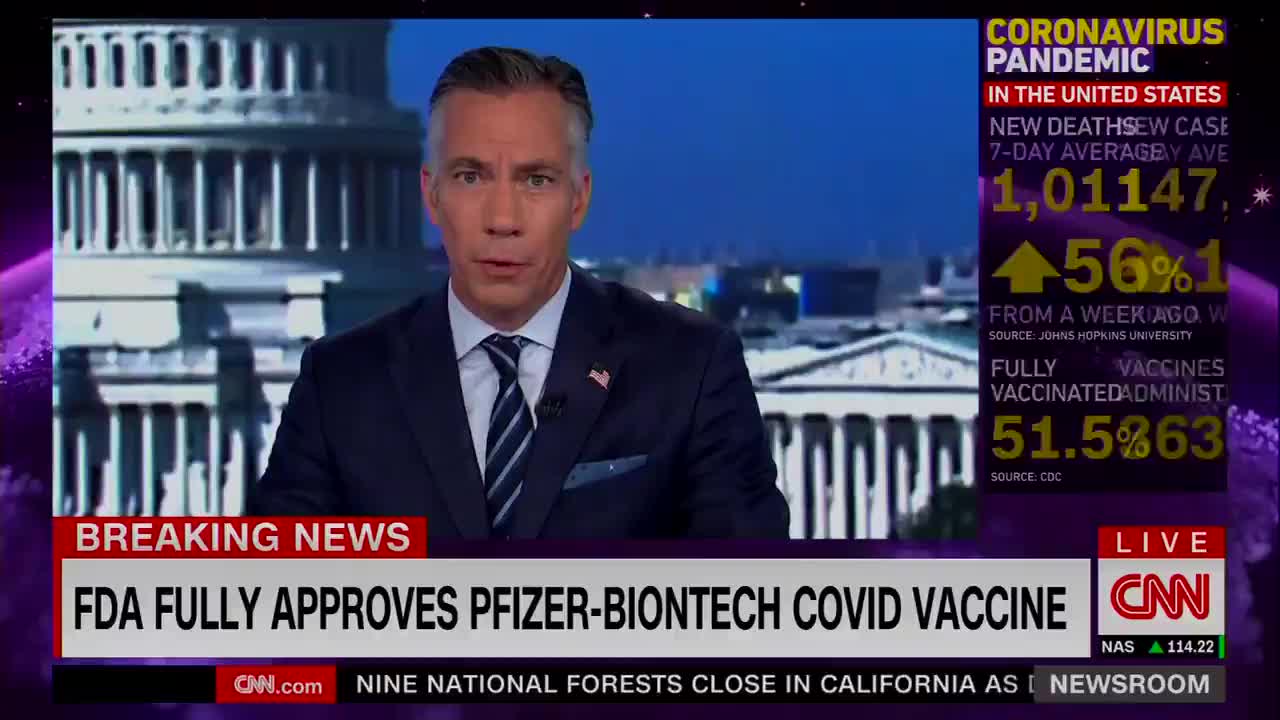
CNN: Full approval of the Pfizer-BioNTech #COVID19 vaccine opens the door for more vaccination-only mandates throughout the U.S.
disclosetv
disclosetv
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
Dennis 1776
@dmrome
23 August, 01:01
In response Dennis 369 to his Publication
I heard it was NOT approved - only the Emergency Use Auth. was extended - is the media LYING ... again???
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
Starry Peacock
@Starry_Peacock
23 August, 01:03
In response Dennis 1776 to his Publication
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
Dennis 1776
@dmrome
23 August, 01:06
In response Starry Peacock to her Publication
TY for confirming !!! YES !!!
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
Leslie Henson
@T_Radioactive
23 August, 01:24
In response Dennis 1776 to his Publication
read the first paragraph
Today, the U.S. Food and Drug Administration approved the first COVID-19 vaccine. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID-19 disease in individuals 16 years of age and older. The vaccine also continues to be available under emergency use authorization (EUA), including for individuals 12 through 15 years of age and for the administration of a third dose in certain immunocompromised individuals.
Today, the U.S. Food and Drug Administration approved the first COVID-19 vaccine. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID-19 disease in individuals 16 years of age and older. The vaccine also continues to be available under emergency use authorization (EUA), including for individuals 12 through 15 years of age and for the administration of a third dose in certain immunocompromised individuals.
Notice: Undefined index: tg1tga_access in /home/admin/www/anonup.com/themes/default/apps/timeline/post.phtml on line 396
The ‘data’ or ‘study’ that the FDA references in the website to aid in FDA approval decision is looking at 20k people
receiving the second dose and 20k receiving a placebo. and monitoring whether or not they get covid 19 symptoms. a large amount did not get symptoms
therefore the conclusion is the vax is effective at preventing serious outcomes & hospitalizations.
🤣🤣🤣🤣🤣🤣🤣
receiving the second dose and 20k receiving a placebo. and monitoring whether or not they get covid 19 symptoms. a large amount did not get symptoms
therefore the conclusion is the vax is effective at preventing serious outcomes & hospitalizations.
🤣🤣🤣🤣🤣🤣🤣
01:40 PM - Aug 23, 2021
In response Leslie Henson to her Publication
Only people mentioned by Kellymalinoski in this post can reply